Summary
Polycystic Ovarian Syndrome (PCOS) is a hormonal disorder marked by elevated androgen levels. Common symptoms include ovarian cysts, irregular menstrual cycles, hirsutism, acne, and obesity. Affecting approximately 8–13% of women of reproductive age, up to 70% of PCOS cases remain undiagnosed globally. It is the leading cause of anovulation and a major contributor to female infertility. Women with PCOS are also at increased risk for diabetes, hypertension, coronary artery disease, anxiety, depression, and endometrial cancer. Infertility, often associated with PCOS, presents a significant emotional and psychological challenge for couples. Moreover, the use of Assisted Reproductive Techniques (ART) can exacerbate mental health issues, particularly anxiety and depression. This review offers a comprehensive exploration of PCOS, with a specific focus on its intersection with psychological disorders.
Keywords
PCOS, androgens, infertility, anxiety, contraception, depression.
Introduction
Polycystic Ovary Syndrome (PCOS) is one of the most prevalent endocrine disorders among women of reproductive age and constitutes a major public health concern [1]. An estimated 8–13% of women in this age group are affected, with approximately 70% of cases remaining undiagnosed [2]. Prevalence is notably higher in certain ethnic groups, who often experience more severe metabolic complications [3]. The disorder’s physical and psychological consequences—especially regarding obesity, infertility, and body image—frequently contribute to mental health challenges and social stigma [4]. PCOS significantly impairs health-related quality of life (HRQoL) and mental well-being, especially among young women. It often persists as a chronic condition beyond adolescence [5]. Contributing factors to psychological distress include noticeable physical changes, obesity, and menstrual irregularities. Although frequently underestimated, the psychological burden of PCOS can be severe, heightening the risk of anxiety, depression, and suicidal ideation [6,7]. Geographic and cultural differences can further influence the psychosocial impact of PCOS. Clinically, it is characterized by oligo- or anovulation and hyperandrogenism, which can lead to infertility and metabolic complications [8]. PCOS increases the risk of reproductive and metabolic disorders such as endometrial cancer, gestational complications, and psychological disturbances. Some symptoms can be mitigated through lifestyle changes, medication, and fertility treatments [9]. Although its exact etiology remains unclear, genetic predisposition and comorbid conditions like type 2 diabetes are significant risk factors [10]. The disorder affects 8–13% of women, with a disproportionately higher prevalence in certain ethnicities, where metabolic complications are also more common [11]. Both the biological and psychological effects of PCOS—especially those linked to body weight, self-image, and fertility—can provoke mental health issues and reinforce stigma [12]. While there is no definitive cure, symptom management is possible. Women experiencing irregular periods, infertility, acne, or hirsutism are advised to consult healthcare providers. Lifestyle interventions, including a nutritious diet and regular exercise, can assist with weight management and reduce the risk of developing type 2 diabetes [13]. Hormonal contraceptives may help regulate menstrual cycles and manage symptoms, while other medications can target acne and excessive hair growth [14]. PCOS may present anatomically as polycystic ovaries or biochemically as hyperandrogenemia. Elevated androgen levels can disrupt follicular development, leading to microcysts, anovulation, and menstrual irregularities [15].
PATHOPHYSIOLOGY IN PCOS
Hormonal Imbalance
PCOS is marked by elevated androgen levels, such as testosterone, which disrupt the normal process of folliculogenesis—the maturation
of ovarian follicles. These hormonal imbalances underlie many of the pathological symptoms observed in women with PCOS [16]. Abnormal
hormonal profiles include imbalances in insulin, growth hormone (GH), ghrelin, LEAP-2, gonadotropin-releasing hormone (GnRH), and the
luteinizing hormone/follicle-stimulating hormone (LH/FSH) ratio, along with elevated androgens and altered oestrogen levels [17]. Such
disturbances are closely linked to a range of metabolic dysfunctions, including insulin resistance, type 2 diabetes, obesity,
infertility, and irregular menstruation [18]. Specifically, increased insulin levels, reduced GH, elevated ghrelin, and leptin
resistance are associated with a greater risk of diabetes and obesity in women with PCOS. Reproductive issues stem from altered
levels of GH, LEAP-2, high LH, increased LH/FSH ratio, elevated androgens, and low oestrogen levels [19].
Insulin Resistance
A large proportion of women with PCOS exhibit insulin resistance, meaning their cells do not respond effectively to insulin. This
results in compensatory hyperinsulinemia and elevated blood glucose levels [20]. Insulin, a central hormone in glucose and lipid
metabolism, also acts as a mitogenic agent. It modulates activity in several organs along the hypothalamic-pituitary-ovarian (HPO)
axis [21]. In steroidogenic tissues such as the ovaries and adrenal cortex, insulin promotes steroid hormone production.
Hyperinsulinemia mimics LH activity and enhances GnRH secretion, driving excess androgen production [22]. Furthermore, insulin
resistance lowers levels of sex hormone-binding globulin (SHBG), a protein that regulates circulating testosterone. Improving insulin
sensitivity through therapeutic interventions can decrease androgen levels and significantly improve PCOS symptoms [23].
Hyperandrogenism
Hyperandrogenism is a hallmark feature of PCOS and manifests in symptoms such as hirsutism, acne, and androgenic alopecia. Around
75–90% of PCOS patients with oligomenorrhea display elevated androgen levels, which often worsen over time [24]. Androgens are
overproduced primarily by the ovaries and, to a lesser extent, the adrenal glands. Elevated levels of free (unbound) testosterone
serve as a reliable marker of hyperandrogenism. Dysfunction in ovarian or adrenal steroidogenesis pathways contributes to this
overproduction [25]. Excess androgens impair follicular maturation, resulting in the development of multiple small ovarian cysts
and menstrual irregularities [26]. During the early gonadotropin-dependent stages of follicular development, high androgen levels
lead to excessive recruitment of primordial follicles and an increase in antral follicles. The hypothalamus secretes GnRH, which
in turn stimulates the anterior pituitary to release gonadotropins [27]. LH binds to receptors in ovarian theca cells, triggering
androgen production, while FSH promotes oestrogen synthesis in granulosa cells by converting androgens into oestrogens necessary
for follicle growth. However, in PCOS, neuroendocrine abnormalities disrupt this balance. Increased GnRH secretion disproportionately
elevates LH levels over FSH, causing an elevated LH:FSH ratio—commonly observed in PCOS. Genetic variations, particularly in the CYP
family of genes, affect steroidogenesis and are closely linked to hyperandrogenism [28].
Genetic and Epigenetic Factors
PCOS has a multifactorial origin involving both genetic and environmental influences. Epigenetic mechanisms—modifications in gene
expression without altering the DNA sequence—also contribute to its pathogenesis. While some familial studies suggest an autosomal
dominant inheritance pattern, the prevailing evidence supports a multifactorial aetiology [29]. The genetic architecture of PCOS is
complex and non-Mendelian, involving multiple loci. Genome-wide association studies (GWAS) and next-generation sequencing have
identified several genes associated with PCOS. Approximately 241 genetic variants are believed to contribute to its development
[30,31]. These include single nucleotide polymorphisms (SNPs) that affect gene expression and function, particularly in genes
regulating steroidogenesis, ovarian theca cell activity, and hypothalamic pituitary signalling. Key genes implicated in PCOS include
INSR, IRS1, GHRL, LDLR, MC4R, ADIPOQ, UCP1, UCP2, UCP3, FTO, PCSK9, FBN3, NEIL2, FDFT1, CYP11, CYP17, CYP21, HSD17, STAR, POR, AKR1C3,
AMH, AMHR2, INHBA, AR, SHBG, LHR, FSHR, FSHβ, SRD5A, GATA4, THADA, YAP1, ERBB2, DENND1A, and FEM1B, among others [32]. Understanding
the genetic and epigenetic underpinnings of PCOS may pave the way for more personalized and effective interventions.
Obesity in PCOS
Obesity is both a clinical feature and a biochemical aggravator of PCOS, particularly among genetically predisposed individuals. Between
38% to 88% of women with PCOS are overweight or obese [33]. Data from the Northern Finland Birth Cohort (NFBC) 1966 demonstrate a
strong correlation between body mass index (BMI) and PCOS features across all life stages [34]. Even modest weight loss (approximately
5%) can lead to significant improvements in reproductive health, androgen levels, and metabolic parameters in women with PCOS. Insulin
resistance is also highly prevalent, affecting 50–90% of individuals with the condition [35]. Despite its frequency, the precise
mechanisms underlying insulin resistance in PCOS remain incompletely understood [36].
Impact of Obesity on the Immune System
Obesity is characterized as a systemic metabolic disorder that disrupts metabolic homeostasis and provokes chronic low-grade
inflammation. A growing body of research has demonstrated a significant correlation between immune system dysfunction and obesity
[37] (Fig. 1). In obese individuals, adipose tissue becomes infiltrated with immune cells, including neutrophils, M1 macrophages, and
T cells. Adipose tissue macrophages (ATMs) play a central role in mediating systemic inflammation. The elevated secretion of monocyte
chemoattractant protein-1 (MCP-1/CCL2) and leukotriene B4 (LTB4) by adipocytes promotes macrophage recruitment and infiltration [38].
Macrophages and adipocytes secrete pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which activate the nuclear factor kappa
B (NF-κB) signalling pathway, further amplifying inflammation. Leptin, an adipokine
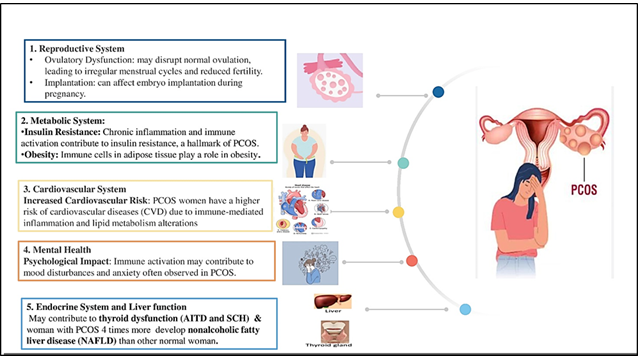
Figure 1. Organ system dysfunction associated with immunological dysregulation in women with PCOS
secreted by adipose tissue, also contributes to immune dysfunction in obesity [39]. Additionally, CD4+ T cells within adipose tissue produce interferon-gamma (IFN-γ). Both macrophages and adipocytes in obese tissue often overexpress MHC class II and costimulatory molecules (CD80 and CD86), functioning as antigen-presenting cells (APCs) that stimulate CD4+ T cell proliferation and differentiation into Th1 cells. These Th1 cells release excessive IFN-γ, worsening the inflammatory environment. Leptin further promotes IFN-γ secretion and MHC class II expression, exacerbating immune imbalance and chronic inflammation in obese individuals [40].
PCOS and Hypertension
Hypertension is characterized by consistently elevated blood pressure levels, impacting a significant portion of the population and leading to severe health complications. In women with Polycystic Ovary Syndrome (PCOS), the prevalence of systemic arterial hypertension (SAH) is notably higher, with studies indicating a 40% increased likelihood of SAH in this group compared to women without the condition [41]. SAH plays a crucial role in the progression of PCOS and associated cardiovascular issues. Clinical research has established a significant link between hypertension and the endocrine system, suggesting that any dysfunction within the endocrine system is likely to correlate with hypertension [42]. In cases of PCOS, endocrine abnormalities are frequently observed, contributing to the development of hypertension. Additionally, insulin resistance and metabolic disorders are prevalent among women with PCOS, further elevating the risk of hypertension [43]. Insulin resistance leads to hyperinsulinemia and an increase in luteinizing hormone (LH) production, which in turn raises androgen levels, resulting in persistently high blood pressure in these patients. Critical clinical studies indicate a high incidence of hypertension among females with PCOS. Therefore, it is essential for women with this condition to regularly monitor their blood pressure to ensure timely management and intervention [44].
Symptoms of PCOS
Symptoms of polycystic ovarian syndrome can vary widely between individuals and may evolve over time. They often emerge without a clear cause. Common manifestations include heavy, prolonged, irregular, or absent menstrual periods; infertility; acne or oily skin; excessive hair growth on the face or body; male-pattern hair loss; and weight gain, particularly abdominal obesity [45]. PCOS significantly increases the risk of type 2 diabetes, hypertension, dyslipidemia, coronary artery disease, and endometrial cancer. Psychological consequences such as anxiety, depression, and poor body image are also prevalent [44]. Symptoms like infertility, obesity, and hirsutism often carry societal stigma, impacting personal relationships, employment, and social functioning [46].
Treatment of PCOS
PCOS is a chronic condition, and available treatments primarily aim to manage symptoms and reduce the risk of long-term complications (Fig. 3). Combination oral contraceptives (COCs), containing both oestrogen and progestin, are the most prescribed medications for regulating menstrual cycles in women with PCOS [47]. COCs also help alleviate hirsutism and acne by suppressing ovarian androgen production. Alternatives such as contraceptive patches and vaginal rings are also available. Progesterone-releasing intrauterine devices (IUDs) are another option for reducing uterine bleeding and protecting against endometrial cancer, although they are less effective for acne and hirsutism [48]. Medications Beyond permanent methods like surgical sterilization, hormonal contraceptives remain the most effective form of birth control. COCs inhibit ovulation by suppressing gonadotropins (FSH and LH), thereby preventing follicular maturation and the mid-cycle LH surge required for ovulation [49–51]. Even in cases where ovulation occurs, these contraceptives may prevent implantation or impair gamete transport. Progesterone also thickens cervical mucus, reducing sperm mobility. Non-contraceptive benefits of COCs include cycle regulation, reduced menstrual flow, and decreased risks of endometriosis and certain cancers [52]. However, COCs are contraindicated in women with a history of thromboembolism, cerebrovascular disease, migraines, liver dysfunction, or cardiovascular issues. Smoking further elevates the risk of adverse cardiovascular events [53]. Forms of oral contraception include
Combination pill: Taken daily for 21 days, followed by 7 days of inactive pills [54]. Extended-cycle pill: Hormonal pills for 12 weeks, followed by 1 week of inactive pills, resulting in quarterly menstruation [55].
Minipill: Progestin-only pills taken daily without a hormone-free interval [56].
Non-Oral Hormonal Contraception Contraceptive patch (Ortho Evra®): Applied weekly to various body sites for three consecutive weeks [57]. Vaginal ring (NuvaRing®): Inserted for three weeks, followed by a one-week break [58]. Male condom: A barrier method worn over the penis before intercourse and disposed of afterward [58].
Emergency Contraception (EC) EC, also called the "morning-after pill," contains high doses of oestrogen and/or progestin to inhibit FSH and LH release, preventing ovulation and implantation. It should be taken within 72 hours of unprotected intercourse, with a second dose administered 12 hours later [59,60].
Hormonal Injections Depot medroxyprogesterone acetate (Depo-Provera®) is administered every three months to inhibit ovulation.
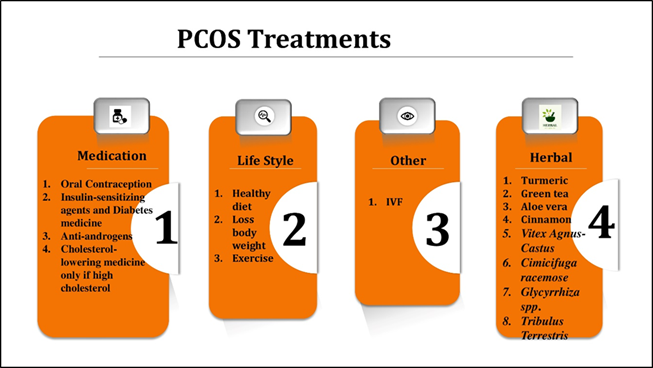
Figure 2. Treatments for PCOS
Insulin-Sensitizing Agents and Diabetes Medications given the role of insulin resistance in PCOS pathogenesis, treatments like metformin and thiazolidinediones (TZDs) help reduce insulin resistance, restore ovulatory function, and lower androgen levels. Metformin improves glucose uptake, decreases hepatic glucose production, and enhances insulin sensitivity in peripheral tissues. It has also been shown to induce weight loss and normalize menstrual cycles without significant lifestyle modifications [61,62].
Lifestyle Modifications
Lifestyle interventions such as improved diet and regular physical activity are first-line treatments for addressing PCOS-related metabolic and reproductive complications [63]. These changes enhance insulin sensitivity, promote ovulation, reduce androgen levels, regulate menstrual cycles, and improve mental health. Even a modest 5–10% reduction in body weight can yield substantial improvements in reproductive function, insulin resistance, and depressive symptoms [64].
Other Treatment Options Hair Removal: Techniques include laser therapy, electrolysis, shaving, and depilatory creams. Assisted Reproductive Technologies (ART): Procedures like in vitro fertilization (IVF) are available for women struggling with infertility [65].
CROSSTALK BETWEEN PCOS AND ANXIETY
Neuroendocrinology offers insights into why anxiety is more prevalent in women with PCOS. The chronic hyperandrogenic, oligo-ovulatory state disrupts the hormonal progression of the menstrual cycle. Persistent anovulation results in deficient progesterone levels and unopposed oestrogen exposure, creating a neurohormonal environment associated with mood instability [66]. Up to 40% of women with PCOS experience anxiety or depression, and some studies report that they are up to six times more likely to suffer from moderate to severe anxiety than the general female population [67]. Meta-analyses show increased odds of depressive symptoms (OR 3.78; 95% CI 3.03–4.72) and anxiety symptoms (OR 5.62; 95% CI 3.22–9.80) in women with PCOS. Factors such as elevated testosterone, insulin resistance, and obesity contribute to these mental health challenges [68]. Oestrogen tends to have excitatory and potentially anxiety-inducing effects on the brain, whereas progesterone exerts a calming, anti-anxiety influence [69,70]. Prolonged exposure to unopposed oestrogen can lead to mood volatility, irritability, and heightened anxiety in susceptible individuals [71]. Treatment with natural progesterone has shown promise in alleviating such symptoms when standard anti-anxiety medications prove ineffective [72]. Anxiety and depression are long known to affect fertility, though exact mechanisms remain unclear [73]. Anxiety becomes pathological when its intensity is disproportionate to the trigger and interferes with daily functioning [74]. According to the World Health Organization, anxiety disorders are projected to become the second leading cause of disability worldwide, following cardiovascular diseases [75,76]. Indian studies reveal a broad range of anxiety prevalence in PCOS patients—from 15.45% to 100% [77]. Infertility is a major psychological stressor for women, giving rise to several anxiety-inducing factors: Societal Pressure and Expectations: Cultural emphasis on motherhood can lead to feelings of inadequacy [78,79]. Loss of Control: Disrupted life plans and bodily autonomy often cause distress [80]. Relationship Strain: Fertility treatments and uncertainty can challenge partnerships [81]. Fear of the Unknown: Uncertainty around treatment outcomes can create chronic anxiety [82]. Hormonal Imbalances: Hormones directly influence mood and emotional regulation [83]. Support from healthcare professionals, mental health counsellors, and PCOS support groups is vital [84]. Additionally, PCOS is associated with a higher prevalence of not only anxiety and depression but also eating disorders, personality disorders, and bipolar spectrum conditions [85,86]. These mental health issues significantly reduce quality of life (QoL) in PCOS patients, and untreated anxiety can delay timely care and worsen outcomes [87].
Future aspects
PCOS is a multifactorial condition with significant long-term consequences. Diagnosis remains challenging due to its heterogeneous nature and evolving diagnostic criteria. The integration of personalized treatment strategies will likely improve outcomes. Early intervention is essential to mitigate comorbidities and improve the quality of life for women of reproductive age experiencing infertility or other complications.
Conclusion
Polycystic Ovary Syndrome (PCOS) is a complex endocrine-metabolic disorder defined by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. It arises from a combination of genetic predisposition, environmental factors, and hormonal imbalances, including insulin resistance and chronic inflammation. Mental health challenges, especially anxiety and depression, are significantly more common in women with PCOS, influenced by hormonal, psychological, and social factors. While current treatments manage symptoms, there remains a pressing need for comprehensive approaches that address both physiological and emotional well-being. Future research should further explore the biological links between PCOS and mental health to inform more targeted, effective therapies.
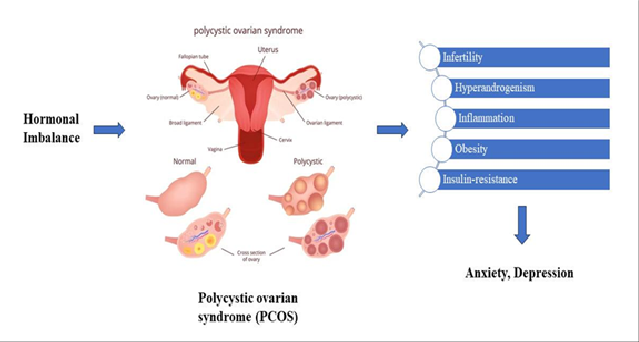
Figure 3. Relation of PCOS with anxiety
References
- E. Joham et al., “Polycystic ovary syndrome,” The Lancet Diabetes & Endocrinology, vol. 10, no. 9, pp. 668–680, Sep. 2022, doi: 10.1016/S2213-8587(22)00163-2.
- J. Yang and C. Chen, “Hormonal changes in PCOS,” Journal of Endocrinology, vol. 261, no. 1, p. e230342, Jan. 2024, doi: 10.1530/JOE-23-0342.
- S. F. Witchel, S. E. Oberfield, and A. S. Peña, “Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls,” Journal of the Endocrine Society, vol. 3, no. 8, pp. 1545–1573, Aug. 2019, doi: 10.1210/js.2019-00078.
- World Health Organization: WHO and World Health Organization: WHO, “Polycystic ovary syndrome,” Jun. 28, 2023. https://www.who.int/news-room/fact-sheets/detail/polycystic-ovary-syndrome
- V. De Leo, M. C. Musacchio, V. Cappelli, M. G. Massaro, G. Morgante, and F. Petraglia, “Genetic, hormonal and metabolic aspects of PCOS: an update,” Reprod. Biol. Endocrinol., vol. 14, no. 1, p. 38, Dec. 2016, doi: 10.1186/s12958-016-0173-x.
- S. Singh et al., “Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics,” J. Clin. Med., vol. 12, no. 4, p. 1454, Feb. 2023, doi: 10.3390/jcm12041454.
- F. Tabassum, C. Jyoti, H. H. Sinha, K. Dhar, and M. S. Akhtar, “Impact of polycystic ovary syndrome on quality of life of women in correlation to age, basal metabolic index, education and marriage,” PLOS ONE, vol. 16, no. 3, p. e0247486, Mar. 2021, doi: 10.1371/journal.pone.0247486.
- J. Bulsara, P. Patel, A. S. Soni, and S. Acharya, “A review: Brief insight into Polycystic Ovarian syndrome,” Endocrine and Metabolic Science, vol. 3, p. 100085, Jun. 2021, doi: 10.1016/j.endmts.2021.100085.
- J. Collée, M. Mawet, L. Tebache, M. Nisolle, and G. Brichant, “Polycystic ovarian syndrome and infertility: overview and insights of the putative treatments,” Gynecological Endocrinology, vol. 37, no. 10, pp. 869–874, Oct. 2021, doi: 10.1080/09513590.2021.1958310.
- C. Dennett and J. Simon, “The Role of Polycystic Ovary Syndrome in Reproductive and Metabolic Health: Overview and Approaches for Treatment,” Diabetes Spectrum, vol. 28, no. 2, pp. 116–120, May 2015, doi: 10.2337/diaspect.28.2.116.
- Y. A. Hajam, H. A. Rather, Neelam, R. Kumar, M. Basheer, and M. S. Reshi, “A review on critical appraisal and pathogenesis of polycystic ovarian syndrome,” Endocrine and Metabolic Science, vol. 14, p. 100162, Mar. 2024, doi: 10.1016/j.endmts.2024.100162.
- P. Sharma, M. Jain, M. Tripathi, M. Sharma, and A. Halder, “An Update on the Genetics of Polycystic Ovary Syndrome: PCOS and Genetics,” JER, pp. 217–240, Jan. 2024, doi: 10.18311/jer/2023/34654.
- D. Anderson, C. M. B. Solorzano, and C. R. McCartney, “Childhood obesity and its impact on the development of adolescent PCOS,” Semin Reprod Med, vol. 32, no. 3, pp. 202–213, May 2014, doi: 10.1055/s-0034-1371092.
- L. Cole and P. R. Kramer, “Chapter 6.8 - Ovarian Cystic Disorders,” in Human Physiology, Biochemistry and Basic Medicine, L. Cole and P. R. Kramer, Eds., Boston: Academic Press, 2016, pp. 219–221. doi: 10.1016/B978-0-12-803699-0.00046-3.
- M. C., N. K. M., U. K. P., J. K., S. K. V., and D. K. P., “Polycystic Ovary Syndrome (Pcos)–An Overview,” Int J Curr Pharm Sci, vol. 10, no. 6, p. 5, Nov. 2018, doi: 10.22159/ijcpr.2018v10i6.30969.
- N. Madnani, K. Khan, P. Chauhan, and G. Parmar, “Polycystic ovarian syndrome,” Indian J Dermatol Venereol Leprol, vol. 79, p. 310, May 2013, doi: 10.4103/0378-6323.110759.
- Y. Li et al., “Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS),” Life Sci, vol. 228, pp. 167–175, Jul. 2019, doi: 10.1016/j.lfs.2019.04.046.
- L.-H. Zeng et al., “Polycystic Ovary Syndrome: A Disorder of Reproductive Age, Its Pathogenesis, and a Discussion on the Emerging Role of Herbal Remedies,” Front. Pharmacol., vol. 13, p. 874914, Jul. 2022, doi: 10.3389/fphar.2022.874914.
- S. Siddiqui, S. Mateen, R. Ahmad, and S. Moin, “A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS),” J Assist Reprod Genet, vol. 39, no. 11, pp. 2439–2473, Nov. 2022, doi: 10.1007/s10815-022-02625-7.
- J. Wang, T. Yin, and S. Liu, “Dysregulation of immune response in PCOS organ system,” Front. Immunol., vol. 14, p. 1169232, May 2023, doi: 10.3389/fimmu.2023.1169232.
- H. E. Cabre, L. M. Gould, L. M. Redman, and A. E. Smith-Ryan, “Effects of the Menstrual Cycle and Hormonal Contraceptive Use on Metabolic Outcomes, Strength Performance, and Recovery: A Narrative Review,” Metabolites, vol. 14, no. 7, p. 347, Jun. 2024, doi: 10.3390/metabo14070347.
- T. Tanbo, J. Mellembakken, S. Bjercke, E. Ring, T. Åbyholm, and P. Fedorcsak, “Ovulation induction in polycystic ovary syndrome,” Acta Obstet Gynecol Scand, vol. 97, no. 10, pp. 1162–1167, Oct. 2018, doi: 10.1111/aogs.13395.
- Talmor and B. Dunphy, “Female Obesity and Infertility,” Best Practice & Research Clinical Obstetrics & Gynaecology, vol. 29, no. 4, pp. 498–506, May 2015, doi: 10.1016/j.bpobgyn.2014.10.014.
- M. Rababa’h, B. R. Matani, and A. Yehya, “An update of polycystic ovary syndrome: causes and therapeutics options,” Heliyon, vol. 8, no. 10, p. e11010, Oct. 2022, doi: 10.1016/j.heliyon.2022.e11010.
- S. Palomba, T. T. Piltonen, and L. C. Giudice, “Endometrial function in women with polycystic ovary syndrome: a comprehensive review,” Human Reproduction Update, vol. 27, no. 3, pp. 584–618, Apr. 2021, doi: 10.1093/humupd/dmaa051.
- U. A. Ndefo, A. Eaton, and M. R. Green, “Polycystic Ovary Syndrome,” Polycystic Ovary Syndrome.
- R. Hart, “PCOS and infertility,” Panminerva Med, vol. 50, no. 4, pp. 305–314, Dec. 2008.
- D. Armanini, M. Boscaro, L. Bordin, and C. Sabbadin, “Controversies in the pathogenesis, diagnosis and treatment of PCOS: focus on insulin resistance, inflammation, and hyperandrogenism,” International Journal of Molecular Sciences, vol. 23, no. 8, p. 4110, Apr. 2022, doi: 10.3390/ijms23084110.
- X. Zeng, Y.-J. Xie, Y.-T. Liu, S.-L. Long, and Z.-C. Mo, “Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity,” Clinica Chimica Acta, vol. 502, pp. 214–221, Mar. 2020, doi: 10.1016/j.cca.2019.11.003.
- M. E. S. Manique and A. M. A. P. Ferreira, “Polycystic Ovary Syndrome in Adolescence: Challenges in diagnosis and management,” Revista Brasileira Ginecologia E Obstetrícia, vol. 44, no. 04, pp. 425–433, Apr. 2022, doi: 10.1055/s-0042-1742292.
- M. Dapas and A. Dunaif, “Deconstructing a Syndrome: Genomic insights into PCOS causal mechanisms and classification,” Endocrine Reviews, vol. 43, no. 6, pp. 927–965, Jan. 2022, doi: 10.1210/endrev/bnac001.
- E. Diamanti-Kandarakis, H. Kandarakis, and R. S. Legro, “The role of genes and environment in the etiology of PCOS,” Endocrine, vol. 30, no. 1, pp. 19–26, Jan. 2006, doi: 10.1385/endo:30:1:19.
- V. Bruni, A. Capozzi, and S. Lello, “The Role of Genetics, Epigenetics and Lifestyle in Polycystic Ovary Syndrome Development: the State of the Art,” Reproductive Sciences, vol. 29, no. 3, pp. 668–679, Mar. 2021, doi: 10.1007/s43032-021-00515-4.
- C. J. Glueck and N. Goldenberg, “Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics,” Metabolism, vol. 92, pp. 108–120, Mar. 2019, doi: 10.1016/j.metabol.2018.11.002.
- L. Barrea, G. Muscogiuri, G. Pugliese, G. De Alteriis, A. Colao, and S. Savastano, “Metabolically Healthy Obesity (MHO) vs. Metabolically Unhealthy Obesity (MUO) Phenotypes in PCOS: Association with Endocrine-Metabolic Profile, Adherence to the Mediterranean Diet, and Body Composition,” Nutrients, vol. 13, no. 11, p. 3925, Nov. 2021, doi: 10.3390/nu13113925.
- H. Cena, L. Chiovato, and R. E. Nappi, “Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists,” The Journal of Clinical Endocrinology & Metabolism, vol. 105, no. 8, pp. e2695–e2709, May 2020, doi: 10.1210/clinem/dgaa285.
- E. Silvestris, G. De Pergola, R. Rosania, and G. Loverro, “Obesity as disruptor of the female fertility,” Reproductive Biology and Endocrinology, vol. 16, no. 1, Mar. 2018, doi: 10.1186/s12958-018-0336-z.
- A. E. Joham, N. Naderpoor, and O. Celik, “Editorial: Mechanisms involved in the development of obesity with PCOS,” Frontiers in Endocrinology, vol. 15, Jul. 2024, doi: 10.3389/fendo.2024.1444299.
- A. P. Snider and J. R. Wood, “Obesity induces ovarian inflammation and reduces oocyte quality,” Reproduction, vol. 158, no. 3, pp. R79–R90, Sep. 2019, doi: 10.1530/rep-18-0583.
- R. Pasquali and C. Oriolo, “Obesity and androgens in women,” Frontiers of Hormone Research, pp. 120–134, Jan. 2019, doi: 10.1159/000494908.
- S. Özkan, Ö. Ç. Yılmaz, and B. Yavuz, “Increased masked hypertension prevalence in patients with polycystic ovary syndrome (PCOS),” Clinical and Experimental Hypertension, vol. 42, no. 8, pp. 681–684, May 2020, doi: 10.1080/10641963.2020.1772815.
- D. Fahs, D. Salloum, M. Nasrallah, and G. Ghazeeri, “Polycystic ovary Syndrome: Pathophysiology and controversies in diagnosis,” Diagnostics, vol. 13, no. 9, p. 1559, Apr. 2023, doi: 10.3390/diagnostics13091559.
- S. Sirmans and K. Pate, “Epidemiology, diagnosis, and management of polycystic ovary syndrome,” Clinical Epidemiology, p. 1, Dec. 2013, doi: 10.2147/clep.s37559.
- O. Osibogun, O. Ogunmoroti, and E. D. Michos, “Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention,” Trends in Cardiovascular Medicine, vol. 30, no. 7, pp. 399–404, Oct. 2020, doi: 10.1016/j.tcm.2019.08.010.
- S. F. Witchel, A. C. Burghard, R. H. Tao, and S. E. Oberfield, “The diagnosis and treatment of PCOS in adolescents: an update,” Current Opinion in Pediatrics, vol. 31, no. 4, pp. 562–569, Aug. 2019, doi: 10.1097/mop.0000000000000778.
- H. F. Escobar-Morreale, “Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment,” Nature Reviews Endocrinology, vol. 14, no. 5, pp. 270–284, Mar. 2018, doi: 10.1038/nrendo.2018.24.
- J. M. Weiss, S. Tauchert, A. K. Ludwig, and K. Diedrich, “Treatment strategies in PCOS patients,” Reproductive BioMedicine Online, vol. 10, pp. 67–74, Jan. 2005, doi: 10.1016/S1472-6483(11)60393-3.
- S. Akre, K. Sharma, S. Chakole, and M. B. Wanjari, “Recent Advances in the Management of Polycystic Ovary Syndrome: A Review Article,” Cureus, vol. 14, no. 8, p. e27689, doi: 10.7759/cureus.27689.
- C. Ep and K. S, “Advances in contraception research and development,” Current opinion in obstetrics & gynecology, vol. 32, no. 6, Dec. 2020, doi: 10.1097/GCO.0000000000000666.
- J. Davis, “Advances in contraception,” Obstet Gynecol Clin North Am, vol. 27, no. 3, pp. 597–610, vii, Sep. 2000, doi: 10.1016/s0889-8545(05)70158-3.
- R. S. Legro et al., “Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline,” The Journal of Clinical Endocrinology & Metabolism, vol. 98, no. 12, pp. 4565–4592, Dec. 2013, doi: 10.1210/jc.2013-2350.
- Hanson, E. Johnstone, J. Dorais, B. Silver, C. M. Peterson, and J. Hotaling, “Female infertility, infertility-associated diagnoses, and comorbidities: a review,” J Assist Reprod Genet, vol. 34, no. 2, pp. 167–177, Feb. 2017, doi: 10.1007/s10815-016-0836-8.
- E. S. Dason, O. Koshkina, C. Chan, and M. Sobel, “Diagnosis and management of polycystic ovarian syndrome,” CMAJ, vol. 196, no. 3, pp. E85–E94, Jan. 2024, doi: 10.1503/cmaj.231251.
- K. M. Hoeger, A. Dokras, and T. Piltonen, “Update on PCOS: Consequences, Challenges, and Guiding Treatment,” J Clin Endocrinol Metab, vol. 106, no. 3, pp. e1071–e1083, Mar. 2021, doi: 10.1210/clinem/dgaa839.
- S. Singh et al., “Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics,” JCM, vol. 12, no. 4, p. 1454, Feb. 2023, doi: 10.3390/jcm12041454.
- Badawy and Elnashar, “Treatment options for polycystic ovary syndrome,” IJWH, p. 25, Feb. 2011, doi: 10.2147/IJWH.S11304.
- eBioMedicine, “Polycystic ovary syndrome: deciphering mechanisms to facilitate management and treatment,” eBioMedicine, vol. 94, p. 104754, Aug. 2023, doi: 10.1016/j.ebiom.2023.104754.
- X. Che, Z. Chen, M. Liu, and Z. Mo, “Dietary Interventions: A Promising Treatment for Polycystic Ovary Syndrome,” Ann Nutr Metab, vol. 77, no. 6, pp. 313–323, 2021, doi: 10.1159/000519302.
- S. Cowan et al., “Lifestyle management in polycystic ovary syndrome – beyond diet and physical activity,” BMC Endocr Disord, vol. 23, no. 1, p. 14, Jan. 2023, doi: 10.1186/s12902-022-01208-y.
- H. J. Teede, “Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome*,” The Journal of Clinical Endocrinology, vol. 108, no. 10, 2023.
- M. Szczuko et al., “Nutrition Strategy and Life Style in Polycystic Ovary Syndrome—Narrative Review,” Nutrients, vol. 13, no. 7, p. 2452, Jul. 2021, doi: 10.3390/nu13072452.
- A. Dokras et al., “Weight loss and lowering androgens predict improvements in Health-Related quality of life in women with PCOS,” The Journal of Clinical Endocrinology & Metabolism, vol. 101, no. 8, pp. 2966–2974, Aug. 2016, doi: 10.1210/jc.2016-1896.
- S. Alesi, C. Ee, L. J. Moran, V. Rao, and A. Mousa, “Nutritional supplements and complementary therapies in polycystic ovary syndrome,” Advances in Nutrition, vol. 13, no. 4, pp. 1243–1266, Jul. 2022, doi: 10.1093/advances/nmab141.
- P. Jin and Y. Xie, “Treatment strategies for women with polycystic ovary syndrome,” Gynecological Endocrinology, vol. 34, no. 4, pp. 272–277, Oct. 2017, doi: 10.1080/09513590.2017.1395841.
- Y. Shang, H. Zhou, M. Hu, and H. Feng, “Effect of diet on insulin resistance in polycystic ovary Syndrome,” The Journal of Clinical Endocrinology & Metabolism, vol. 105, no. 10, pp. 3346–3360, Jul. 2020, doi: 10.1210/clinem/dgaa425.
- J. V. Kolhe, A. S. Chhipa, S. Butani, V. Chavda, and S. S. Patel, “PCOS and Depression: common links and potential targets,” Reproductive Sciences, vol. 29, no. 11, pp. 3106–3123, Oct. 2021, doi: 10.1007/s43032-021-00765-2.
- P. Chaudhari, K. Mazumdar, and P. D. Mehta, “Anxiety, Depression, and Quality of Life in Women with Polycystic Ovarian Syndrome,” Indian Journal of Psychological Medicine, vol. 40, no. 3, pp. 239–246, May 2018, doi: 10.4103/IJPSYM.IJPSYM_561_17.
- L. G. Cooney and A. Dokras, “Depression and Anxiety in Polycystic Ovary Syndrome: Etiology and Treatment,” Curr Psychiatry Rep, vol. 19, no. 11, p. 83, Nov. 2017, doi: 10.1007/s11920-017-0834-2.
- G. Herzog, “Menstrual disorders in women with epilepsy,” Neurology, vol. 66, no. 66_suppl_3, Mar. 2006, doi: 10.1212/WNL.66.66_suppl_3.S23.
- L. Damone, A. E. Joham, D. Loxton, A. Earnest, H. J. Teede, and L. J. Moran, “Depression, anxiety and perceived stress in women with and without PCOS: a community-based study,” Psychol. Med., vol. 49, no. 09, pp. 1510–1520, Jul. 2019, doi: 10.1017/S0033291718002076.
- Dokras, “Mood and anxiety disorders in women with PCOS,” Steroids, vol. 77, no. 4, pp. 338–341, Mar. 2012, doi: 10.1016/j.steroids.2011.12.008.
- W. K. Almeshari, A. K. Alsubaie, R. I. Alanazi, Y. A. Almalki, N. Masud, and S. H. Mahmoud, “Depressive and Anxiety Symptom Assessment in Adults with Polycystic Ovarian Syndrome,” Depression Research and Treatment, vol. 2021, pp. 1–8, Apr. 2021, doi: 10.1155/2021/6652133.
- Ambreen, A. Sheikh, N. Faryad, S. Batool, and F. U. A. Ahmed, “Depression and Anxiety in Women with Polycystic Ovary Syndrome and Its Biochemical Associates,” Journal of South Asian Federation of Obstetrics and Gynaecology, vol. 8, no. 1, pp. 44–47, Mar. 2016, doi: 10.5005/jp-journals-10006-1384.
- P. Gdańska, E. Drozdowicz-Jastrzębska, B. Grzechocińska, M. Radziwon-Zaleska, P. Węgrzyn, and M. Wielgoś, “Anxiety and depression in women undergoing infertility treatment,” Ginekol Pol, vol. 88, no. 2, pp. 109–112, Feb. 2017, doi: 10.5603/GP.a2017.0019.
- J. Zhuang, X. Wang, L. Xu, T. Wu, and D. Kang, “Antidepressants for polycystic ovary syndrome,” Cochrane Database of Systematic Reviews, vol. 2013, no. 6, May 2013, doi: 10.1002/14651858.CD008575.pub2.
- R. Wang, R. Zhao, and D. Qin, “Depression in polycystic ovary syndrome: Focusing on pathogenesis and treatment,” Frontiers in Psychiatry.
- P. Gdańska, E. Drozdowicz-Jastrzębska, B. Grzechocińska, M. Radziwon-Zaleska, P. Węgrzyn, and M. Wielgoś, “Anxiety and depression in women undergoing infertility treatment,” Ginekol Pol, vol. 88, no. 2, pp. 109–112, Feb. 2017, doi: 10.5603/GP.a2017.0019. S. A. Buriro, S. A. Memon, Z. Iqbal, I. Chandio, H. Bano, and D. S. Thebo, “Analysis of Anxiety, Depression and Perceived Stress in Women With Polycystic Ovary Syndrome (PCOS),” 2023.
- Ethirajulu et al., “Insulin Resistance, Hyperandrogenism, and Its Associated Symptoms Are the Precipitating Factors for Depression in Women With Polycystic Ovarian Syndrome,” Cureus, Sep. 2021, doi: 10.7759/cureus.18013.
- T. Jannink et al., “Anxiety, depression, and body image among infertile women with and without polycystic ovary syndrome,” Human Reproduction, vol. 39, no. 4, pp. 784–791, Apr. 2024, doi: 10.1093/humrep/deae016.
- A. Deeks, M. E. Gibson-Helm, and H. J. Teede, “Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation,” Fertility and Sterility, vol. 93, no. 7, pp. 2421–2423, May 2010, doi: 10.1016/j.fertnstert.2009.09.018.
- L. Doretto, F. C. Mari, and A. C. Chaves, “Polycystic Ovary Syndrome and Psychotic Disorder,” Front. Psychiatry, vol. 11, p. 543, Jun. 2020, doi: 10.3389/fpsyt.2020.00543.
- P. Dybciak, E. Humeniuk, D. Raczkiewicz, J. Krakowiak, A. Wdowiak, and I. Bojar, “Anxiety and Depression in Women with Polycystic Ovary Syndrome,” Medicina, vol. 58, no. 7, p. 942, Jul. 2022, doi: 10.3390/medicina58070942.
- Ethirajulu et al., “Insulin Resistance, Hyperandrogenism, and Its Associated Symptoms Are the Precipitating Factors for Depression in Women With Polycystic Ovarian Syndrome,” Cureus, Sep. 2021, doi: 10.7759/cureus.18013.
- A. L. Damone, A. E. Joham, D. Loxton, A. Earnest, H. J. Teede, and L. J. Moran, “Depression, anxiety and perceived stress in women with and without PCOS: a community-based study,” Psychological Medicine, vol. 49, no. 09, pp. 1510–1520, Aug. 2018, doi: 10.1017/s0033291718002076.
- S. Alur-Gupta and A. Dokras, “Considerations in the Treatment of Depression and Anxiety in Women with PCOS,” Seminars in Reproductive Medicine, vol. 41, no. 01/02, pp. 037–044, Mar. 2023, doi: 10.1055/s-0043-1777720.
- D. Rodriguez-Paris et al., “Psychiatric disorders in women with polycystic ovary syndrome,” Psychiatria Polska, vol. 53, no. 4, pp. 955–966, Aug. 2019, doi: 10.12740/pp/onlinefirst/93105.
- C. Brutocao, F. Zaiem, M. Alsawas, A. S. Morrow, M. H. Murad, and A. Javed, “Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis,” Endocrine, vol. 62, no. 2, pp. 318–325, Jul. 2018, doi: 10.1007/s12020-018-1692-3.